From a paper delivered during the Dec. 10 webinar, “Health and Economic Outlook for 2021—Sparking Hope: Will a Vaccine see an end to the Pandemic?” conducted by the Philippines Graphic-BusinessMirror, in partnership with the Pharmaceutical & Healthcare Association of the Philippines (PHAP).
This morning, I will discuss the Philippine COVID–19 roadmap. We will also discuss some of the highlights of the government’s vaccine development program and present the Philippine COVID–19 National vaccine roadmap. We will also discuss the ongoing public and private partnership that we have undergone lately.
Today I am going to cover “The State of Vaccine Development–The complex journey.” There are five points that I want to share with you today. The first one is the different stages of the vaccine clinical trials; Second, the history of the development of vaccines; Third, the impact of vaccines on public health; Fourth, the recent vaccines under development and what are expected to see within the coming five to 10 years; and Fifth, the most important topic that everyone wants to hear—the COVID–19 vaccine development, before I will have my conclusion.
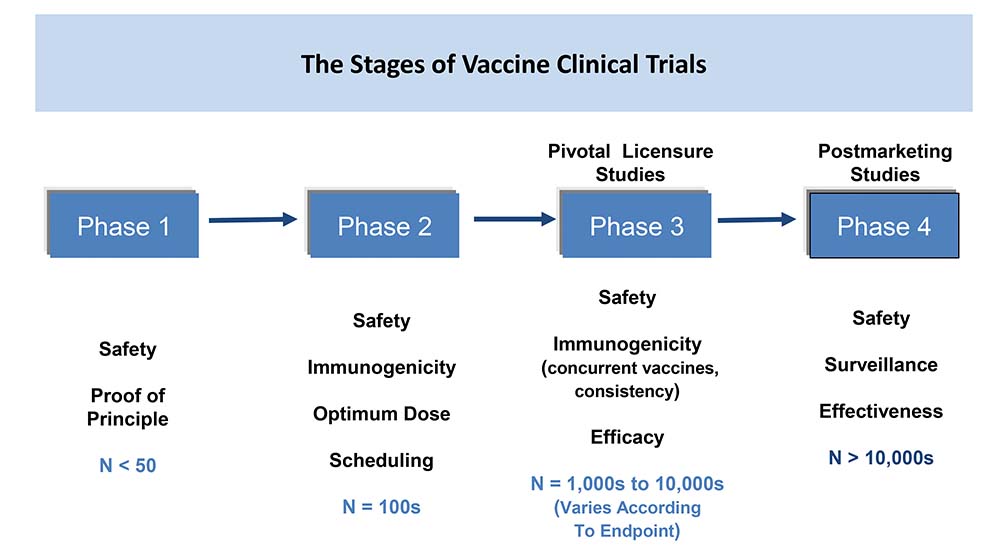
We all know that there are four phases of vaccine clinical trials. Now in the vaccine clinical study, usually the Phase 1, after you have finished the pre–clinical and then you go to the Phase 1, this is usually a small study where we look for the safety and the proof of principle and usually it is done on a small number of subjects from 50 to 100 individuals.
Once this is finished, we move to Phase 2. So, these are like studies where we look mainly to the safety, to the immunogenicity, as well as the optimum dose. So, this is where we start to look at what would be the optimum dose we can use to induce that immunogenicity as well as safety profile to this population. This is also the study where we start to study the different schedules of the vaccination and usually we have numbers of about 100 to 200.
Now once we finish the Phase 2, we move to the Phase 3, which is the gestation phase or the gestation study. This is the pivotal study which is used for the licensing and this is usually where we look for a larger scale of population, sometimes from 1,000 up to 10,000 depending on the vaccine and the disease area. And this is where we look on the immunogenicity versus the comparator or versus the correlate of protection. And of course we also look into the safety and the efficacy of the vaccine.
It is also important to know that usually we also look into the immunogenicity comparing it from the other vaccines or from the correlate of protection. Once we finish and we realize the vaccine, the phase four or the post market studies, this is an important phase because this is where you want to demonstrate the real safety and effectiveness of the vaccine from the population based on the surveillance. And, this is different because in the clinical studies, you have a strict inclusion and exclusion criteria and this is where you have the best patients’ profile.
But when you go to the post marketing, this is the real life experience of the civilian population. This is where we want to demonstrate the surveillance and the effectiveness and these types of studies can contain from 10,000 up to even millions of the population.
VACCINE DEVELOPMENT
There was a development in the vaccine happening over the years and we started from the empirical approach, from the normal vaccine like the BCG, like diphtheria, and pertussis. And then we started to move to Recombinant DNA vaccine like Acellular pertussis, Hepatitis B, and then we moved to the next phase when we started to have Glycoconjugation and the conjugated vaccine. This is the era when we had the conjugated pneumococcal, conjugated meningococcal and this has been a major dramatic development in the vaccine.
We are moving forward now to the Reverse Vaccinology though some of the vaccines are still in the clinical trial like C difficile. And, on the next generation, now we have the DNA and RNA vaccine which now started to be, there was a number of clinical trials in the Phase 1 and Phase 2 but now with COVID–19, we started to see vaccines now in the market.
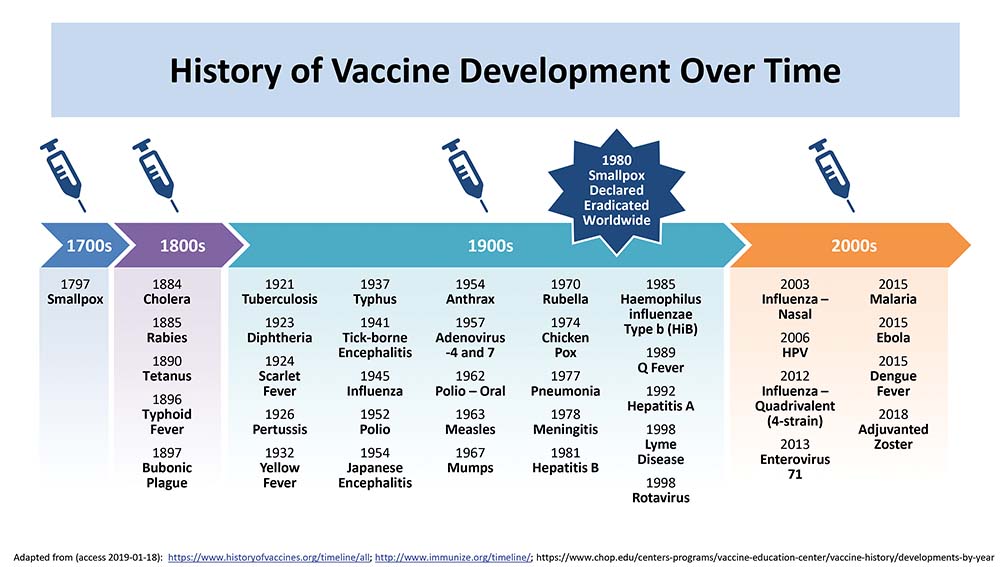
Just to give you a snapshot of what has happened in the last 200 years—since the early development of the vaccine, and since we started with the small pox which was a disease killing millions of the population and the vaccination was one of the areas to control this disease.
Then in the 1800, there was an introduction of other vaccines like cholera, rabies, tetanus and then in 1921 until 2000, there was an introduction of a lot of vaccines. I am not going to go through all of them, but if you look at the last 20 years, I think the introduction of the Haemophillus influenza B vaccine, the pneumococcal, the meningococcal, caused a major management of a disease which was causing millions of deaths.
In the last 20 years, there was an introduction of a number of influenza vaccines and there were a number of other influenza vaccines that were actually in preparation and development. There was also the malaria, there was also the ebola, dengue fever. All of these diseases caused a lot of morbidity and mortality.
PUBLIC HEALTH
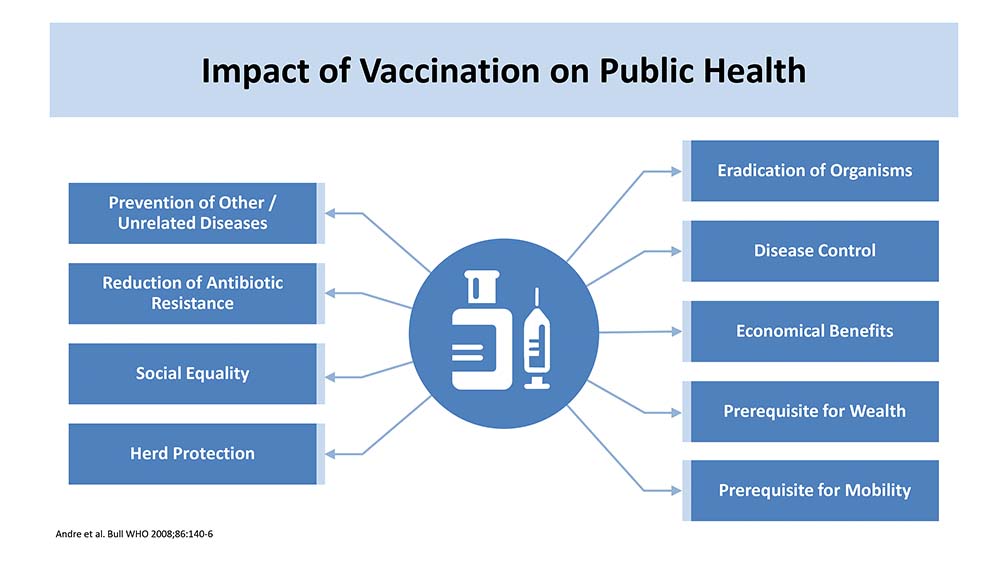
This is one of the important slides (SLIDE 3) because this summarizes the impact of vaccination on public health. And the impact of vaccination is not only preventing or reducing the instance of the disease. Of course, one of the most important elements in the impact on public health is they get eradication of this disease or a specific organism or at least limit the spread of transmission by this organism.
The other important public element is the control of the disease. And from all the vaccines that I have shared, there is plenty of evidence showing that vaccines reduce the instances and prevalence of the disease.
It reduces the burden of hospitalization in a lot of vaccines and also reduces the mortality, which is one of the most important elements, especially when it comes to a disease like COVID–19 or pediatric diseases.
Now we go to the economic and cost effectiveness analysis and that is also one of the most important. In a lot of these vaccines, there is a lot of evidence confirming that when you give the vaccine, you are not only controlling the disease, but you also have the cost effectiveness impact on public health. And a lot of these vaccines also has cost saving impact to the community.
It is important that vaccination reduce the morbidity and allow more mobility. A lot of these vaccines have a lot of impact when we come to the morbidity of the vaccine.
Another important aspect that the vaccine has played a major role in some of the diseases is the reduction of antimicrobial resistance. This is important because in Asian countries, we have one of the highest antimicrobial resistance and a lot of introduction of public health programs for antibiotic stewardship program. And the vaccine played a major role here in the reduction and in the impact to public health.
And then, there is the impact on society and one of the most important impacts is the herd protection. This is important because we give the vaccine to a specific population in the community and then we reduce the burden of the disease and the solution to the other age groups in the community. And we noticed from the early days when we have the pneumococcal vaccine, it was given to children under five years old and then we started seeing a reduction in the pneumococcal disease in the elderly, aged 65, so this is also important.
Vaccines will play a major role in reducing antimicrobial resistance and this is through three main pathways. The first is existing vaccines can prevent infectious diseases whose treatment would require antimicrobial medicines. The other point is that existing vaccines can reduce the prevalence of primary viral infections, which are often inappropriately treated with antibiotics, and which can also give rise to secondary infections that require antibiotic treatment. Third is the development and use of new or improved vaccines can prevent diseases that are becoming difficult to treat or are untreatable owing to antimicrobial resistance.
And when you look at the same report, you will see that the World Health Organization (WHO) has identified a number of pathogens that play a major role in antimicrobial resistance. Now luckily, we now have vaccines for pneumococcal disease and the vaccine has played a major role not only in controlling the disease but it is also evident based on reducing the antimicrobial resistance. And when you look to the rest of the pathogens, there is a lot now of effort in Phase 2 and Phase 3 of developing vaccines that within the coming five to 10 years, some of these pathogens can be prevented by vaccination.
Now this is another impact on the public health and this is from the United States Center for Disease Control (CDC) data and these are the cases which can be prevented by vaccination in 10 years in the United States.
You can see the long list of diseases and you can see the number by thousands whether those you can reduce illness by giving the vaccine in the US as well as hospitalization and more importantly, the mortality. And I can add to that, when you look at the cost effectiveness analysis, and the reduction of cost saving and cost effectiveness to the public health.
In the coming years, there will be a lot of development of new vaccines so we started to see the conjugation. There is the vaccines that use protein conjugations, there is the DNA and viral vector. There is the RNA vaccines and we got a lot of these vaccines usually in Phase 2 and Phase 3. And when you look at the Jordon Report you will find that there are at least vaccines working now in the trial against 35 different diseases in the Phase 3. And, we will see in the coming years, vaccines against diseases like CMV, dengue, HIV, other diseases like Staph aureus, will come and we will see it and expect it in the next five to 10 years.
COVID–19 VACCINES
Now this is the last point that I want to share with you about the vaccines for the COVID–19 development. Look now at the numbers of the graph you see on the lower part of the slide. This is the latest update from the WHO report on the 2nd of December. We have 163 molecule candidate vaccines in three clinical studies. And there are 51 candidates in the different phases of Phase 1, 2 and 3. There are 23 in Phase 1, 12 in Phase 2 and 13 in Phase 3.
And I also want to mention that because of the COVID–19 pandemic, we have adopted the methodology of doing the studies so a lot of these candidates, they have Phase 1 and Phase 2 together or Phase 2 and Phase 3 together because of the need to have an adapted methodology and to accelerate and we have now one approved vaccine and actually this is not an updated when I developed the slide one week back because we have now one the MRA the Pfizer BioNTech vaccine which you heard, it was approved in the United Kingdom but in the last few days, there was also another two approvals from Bahrain and yesterday Canada. And today there will be a big meeting from the Biologic and Vaccine Panel Advisory Board where they are going to review the Pfizer BioNTech vaccine.
Now, one important thing that in the development which I mentioned in the first slide is that the development of a vaccine can take from three to eight years and when you look at regulatory review, it usually takes one to two years of review even with the priority review with the FDA, it will take six to eight months.
This has been completely changed because of the pandemic and we have now this candidate in Phase 3. Most of the clinical studies started in February and March after China announced the genetic sequence of the spike protein so in less than a year, we could accelerate the development to have a number of candidates in Phase 3.

This is the slide (SLIDE 4) showing the different vaccine platforms. There are a number of candidates on different platforms for COVID–19 vaccines that are now under development. So on the right side, there are those who are working on nucleic acid like the DNA and the RNA and there are a number of vaccines in each one of the DNA and RNA and even in the RNA, there is a different platform so there is the modified RNA, there is the routine RNA, there is the self–amplifying RNA. And even in the modified RNA, there are vaccines working on different targets of the spiked proteins. So some of them are working on the rapid binding domain, some of them in the spike protein and some of them on the food spike protein and there is a different methodology and you can see on the left side these are the new molecules which you can use the LMP or lipid manual particle which the nubicle composed of the genetic sequence of the RNA and surrounded by the lipid particle and this is the vehicle that can carry the genetic sequence of the MNRA to the body.
There are also vaccines that are working on the protein based or the protein subunit and the RA vaccine, this is the vaccine like Pfizer and Moderna. These are also the protein based and the protein subunit there are a lot of other companies like Novavax. And there is also the old platforms of the viral vector whether that is replicating or non– replicating, and there is the live attenuated by vaccine and the non live attenuated vaccine. So there are many different platforms and each one targets different parts. Now, it is important to mention that all of these different platforms target the spiked protein so the objective is to get antibodies against the spiked protein and by targeting the spiked protein, either by targeting the rapid body domain or the S Protein or the food spy protein.
REGULATIONS

Now this is important and this is the regulatory authority evaluating the vaccine candidate. And before I go to this slide, I just want to remind everyone that this is a challenging situation when we are developing the vaccine. We rather have it in the last 200 years and the reason for that is two important things. We don’t know up until now everything about the virus. We don’t know everything until now about the COVID–19 disease or the SARS Coronavirus and we are just learning every day.
The other important thing is we don’t have any corelate of infection for this vaccine like other vaccines when we were developing. So, it was important to identify a number of parameters that can allocate, allow us to evaluate the vaccine and the vaccine now is evaluated under these important parameters:
First is vaccine efficacy. So in phase 3, we answer how much the vaccine can reduce the incidence of COVID–19 infection before and after the vaccination.
The other one is the ability of the vaccine to produce neutralizing antibodies. Now one important thing I just want to share is that because we don’t have any co–related protection with the COVID vaccine, the companies now are are utilizing antibodies. They are relying on looking for the neutralizing antibody by geometric means and comparing it with the antibody, from convalescent, from those who got infected by the disease and the threshold here is to have this neutralizing antibody at least as equal or higher from the neutralizing antibody of the convalescent or those who got interaction.
The third important element is the impact of the vaccine on the cellular response. The impact of the vaccine not only to produce neutralizing antibodies but if it has an impact on the CD 4 and CD8 plus and this is important for two reasons. One of them is that if there is a way to the Immune response, this impact on the cellular response might give us a longer term of protection and the other thing is it has to be verified but CD 4 and CD 8 cells play a major role in the disease transmission of COVID 19.
The important other element is the durability. How long can the protection last with this vaccine? I just want to let you know that this is something we need to wait another one and two years because all of the vaccines that are under development actually started the Phase 3 in June or July of this year. So we might have to wait for a little bit for the answer to this question, in one or two years.
But the other important element to the regulatory authorities is the safety profile and this is one of the most important elements. In the safety profile, there are a number of elements and I just want to share with you that some of the agents like the US, when they were going to have the urgent authorization approval for the COVID–19 vaccine, they added a number of additional requirements to these vaccines. So from safety `profile, they requested to have the safety profile in the different age groups. They requested to have some analysis for those under 65 and those above 65. They requested to have the safety analysis for the different age groups, for the different races, the different ethnic groups In the United States and others and also required them to have at least 50% of the population in the Phase 3 to be followed for two months of safety monitoring after the second dose. And they also added additional parameters for efficacy and the threshold is 50% efficacy but they accepted to go to 30% to the lower part. And this is now a major breakthrough when we look at the vaccines, the RNA vaccines where the BioNTech Pfizer vaccine is the efficacy of 95% of the Moderna vaccine with 94% or the Oxford vaccine with 90% after the first dose or 62% after the second dose. Their storage and distribution is also one of the evaluation of the vaccine candidate. So, this is the criteria the regulatory agencies are looking to how fast they can evaluate the COVID–19 vaccine.
ACCELERATED DEVELOPMENT

The development of vaccines over the last years has led to elimination of a lot of major killing diseases like smallpox and a lot of reduction in morbidity and mortality of a lot of invasive diseases.
The WHO has stated earlier that three years back malaria and pneumococcal disease are the disease of highest priority for prevention. Now probably we are moving to a new area where the COVID–19 is one of the most important diseases.
Of course, the introduction of new technology like conjugated vaccines had made a major impact on the reduction of invasive diseases meningococcal, pneumococcal diseases. There are a lot of developments in new vaccines which we are going to see in the coming five to 10 years.
Now because of the COVID–19 pandemic, it became major and important to accelerate the development and the introduction of this vaccine because it is one of the most important element that can counter the disease in addition to the therapeutic element. There are now a number of vaccines that have been under development and the good news by the end of 2020 and early next year, we might have at least five to 10 COVID–19 vaccines in the market.


