Three million children’s lives saved each year. This is the greatest impact of vaccines in our world today.
Vaccines now account for 95% of global health and the World Health Organization (WHO) has ranked them second only to clean water among the best ways of preventing infectious diseases.
Aside from prolonging life expectancy, reducing mortality and saving trillions of dollars in curative health costs, vaccines enhance and improve the life and welfare of humankind.
More than 200 years of development, experimentation and discovery now define the success, failure and indispensable niche of vaccines on human society. Long before antibiotics and other life saving drugs were discovered and developed, vaccines have effectively reduced deaths in the world and they are definitely relied upon tomorrow to address infections that may become resistant to antibiotics in the future.
By far, vaccines and vaccination are among the best, most important and significant weapons against man’s most debilitating and fatal diseases.
HISTORY OF VACCINES

English physician Edward Jenner is often referred to as the “Father of Immunology,” having discovered the smallpox vaccine in 1796. Smallpox has unknown origins with claims that the malady has been in existence since the Egyptian empire around the 3rd century BCE on recounts of rashes resembling smallpox in three mummies. (1)
Prior to 1960, small pox was ranked with malaria and tuberculosis as the main causes of death due to infectious disease. (2)
Smallpox is due to the variola virus and belongs to the Poxviridae family, having monkeypox, cowpox and vaccinia virus as members that is known to infect humans.
Significantly enough, we, homo sapiens are the only sources of the virus. Transmission is via infected respiratory droplets with rare reports of airborne and contact transmission from infected lesions or infected fomites. (3) Infection may lead to several complications such as death, severe scarring or in rare instances, blindness. (4)
Knowing the devastating characteristic of this particular virus family and how they have killed hundreds of millions of people in the past, medical science responded. Proponents focused on how vaccine and immunization have amazingly eradicated smallpox today. This success story is often considered and appreciated as “poster girl,” sparking the development of more life-saving vaccines and will hopefully provide the reader, a deeper understanding on the value of vaccination.
Before the discovery of vaccination, medical practitioners have already experimented on introducing a pathogen or antigen into a living organism for the purpose of stimulating the production within them, of so called antibodies.
The fact that living organisms by nature find ways to protect themselves from external harm and intrusion opened doors to the study of these antibodies production within a living thing.
The process of inoculation whose practice dates back to earliest times ensued and was later termed as variolation; predecessor of modern vaccination. Conflicting historical accounts spark debate as to exactly where and when it started and point to a case of a tug of war between ancient societies of China and India. Nevertheless, one can only marvel as to the lengths that were taken to find the right tool to combat and in this instance, prevent disease.
Arthur Boylston, in his article on the origins of inoculation made mention of several practices which ranged from blowing smallpox scabs into the nostrils of the child, rubbing fluid from the lesion into a cotton plug and stuffing it into the nostril, rubbing scabs into the skin, puncturing the skin with a needle contaminated with smallpox matter and even trading goods for smallpox material referred to as “buying the pocks” in Scotland and mainland Europe. There were also accounts of having children wear smallpox-infected clothes or wrapping wool contaminated with smallpox material on their wrists. (5)
These methods were actually reflective of the basic principles of immunology. In pursuit of cures and disease prevention, people back then dared to invite actual exposure hoping that illness may ensue and eventually trigger the human body to automatically protect itself—producing antibodies to cure itself of the disease and/or prevent such from recurring. The first practices were further bolstered by positive results observed on those individuals who survived smallpox infection and successfully became immune to the disease.
Pioneering Dr. Jenner worked around this principle at the turn of the 18th century. He found out that farm milkmaids who were exposed to cowpox then and survived, never suffered from smallpox. He placed his theory to test. He daringly inoculated pus from a cowpox lesion (obtained from a milkmaid) into the arm of his gardener’s eight-year-old son James Phipps. Then he repeatedly exposed the subject child to smallpox. It was a resounding success. Phipps never developed the disease.
The daring doctor then went on to document his findings along with 13 other cases but unfortunately, his paper was rejected by the Royal Society of London who dismissed his work due to insufficient evidence. A sorry turn of events for the history of vaccination. However, Dr. Jenner persisted and this persistence has led to the eventual eradication of smallpox, “considered as the biggest achievement in international public health.” (1)
The term vaccination was actually derived from variolae vaccinae, which means “smallpox of the cow” coined by Dr. Jenner and adopted by Pasteur as a tribute to Jenner for any protective inoculation. (6)
Since the advent of the smallpox vaccine, several milestones for vaccine discoveries have been described during the more than 200 years of continuous research and development to improve the safety, efficacy, and effectiveness of vaccines.
The Nobel Prize for immunology and vaccines was awarded to John Enders and Jonas Salk for Polio while Max Theiler received the award for Yellow Fever vaccine in 1951. Their combined efforts have brought cure and prevention to unimaginable millions of human lives.
VACCINE DEVELOPMENT
The process of vaccine development is long, tedious and rigorous. Needless to say it comes, too, with a very, very huge price tag. Coming from its humble theoretical and experimental origins, the development has had more misses than successes throughout its history. The wisdom of its development lies in the process itself namely: Pre-trial, Clinical trial in all three phases, and Marketing and Post-Marketing Surveillance.
Pre-Clinical Phase of Vaccine Development: It all begins in a laboratory. The foremost task is to determine which part of a living organism subject will stimulate its natural and inherent immune system to respond to the introduction of a foreign body or substance thereby triggering a reactive production of bodily countermeasures.
As in the case of humans, the Pre-Clinical phase to develop a specific vaccine usually takes one to five years to determine and highly dependent on whether bacterial, viral, or fungal infections are involved.
The differences among these lie on the various cellular or characteristic structures that define each kind. The whole body may be used or a cellular part thereof may be selected to design the experiment and observe how the introduction of a foreign substance will affect the subject.
Scientists have found that bacterial organisms are much easier to work with than viruses, owing to the earlier approval and mass use of vaccines for tuberculosis, diphtheria, tetanus and pertussis vaccines.
Viral vaccines take longer to perfect such as those for polio, measles and yellow fever. Viruses, unlike bacteria, are incomplete organisms that live inside the cells being intricate intracellular parasites to the human body.
Scientists must first identify which unimaginable numbers of live cells in the body will be used to grow the viruses. Primate-monkey and similar animal kidney cells and egg cells have traditionally been used for the purpose. Once the study and experimentation produces significant evidence that a specific material prevents growth of the disease-causing organism, test animals—mice, rats, guinea-pigs, hamsters and then primates: monkeys are then lined up for experimentation.
In succeeding animal laboratory experiments, the disease-causing organisms are then injected or administered to the vaccinated laboratory animal to see if they will get sick and infected. These animal experiments may last for another three to five years or be extended for as long as there is sufficient evidence that the vaccine material can prevent the disease and will not be harmful.
Once it is established that this material can prevent the disease, is not harmful to the subject, and presents no safety concerns for humans, then the Human Clinical Phase of the vaccine development can begin.
Clinical Phases of Vaccine Development: Human vaccine trials are composed of three, namely:
Phase 1: This is devoted to determining the safety of the vaccine when administered to humans. The number of subjects involved can range from 20-80 depending on the initial findings on animal models. Subjects may be divided into two or three groups assigned with a different dose of the same vaccine to check for any adverse event and reaction at each different dose level, as well as to find the safest dose, which will produce the least or no significant adverse event or reaction whatsoever. Should there be any serious or severe reaction on any of these subjects, the vaccine may no longer be brought to Phase 2. This phase may last for two to six months.
Phase 2: Immunogenicity or the ability to produce antibodies known to prevent a disease from developing is the main objective of the second phase of the human clinical trial. It usually enrolls from 100-800 or less than 1,000 subjects and may last for three to twelve months. It builds on the positive results of Phase 1 and needs to show outright evidence of safe introduction and that adequate amounts of antibodies production are observed as a result. A good percentage or acceptable number of subjects must reach the level of antibodies needed for adequate protection of the subjects. This could be from 50-99% of subjects; the higher the percentage, the higher the significance, the more effective is the vaccine.
Should there be any safety concern or adverse events observed, or the level of protection sought is not achieved, referred to in medical circles as immunogenicity, the vaccine is abandoned and may no longer be brought to Phase 3. It is also usual practice to do a randomized double blind trial, placebo-controlled clinical trial even in Phase 2 to maintain an unbiased and standard conduct of the study. By this is meant, a comparison will be made between or among two or more groups, one or two groups being given the study vaccine in one or two doses, another group without the vaccine (Placebo or another commercially available vaccine).
These groups will be chosen randomly through a computer or other ways, without the subject or the investigator knowing what the subject received. All subjects will undergo the same observation procedures and only when the study has been terminated will the investigator and the subjects be informed of the results. Only if the Phase 2 clinical trial shows evidence of safety and good immunogenicity will the vaccine proceed to Phase 3. In some cases, a Phase 2 trial may have subjects continue to Phase 3 trials with additional subjects to satisfy the requirements for a Phase 3 trial.

Phase 3: This phase involves a significant number of subjects from 1,000 to as much as 20,000. In the case of the current COVID-19 vaccine trial, some 30,000 subjects are to be recruited for every vaccine under study. Some vaccine Phase 3 human trials have even gone as far as 70,000, as in the rotavirus vaccine studies.
The number of subjects required depends on the incidence and cases present in the community or country. Phase 3 is undertaken with these many subjects after having proven the safety and immunogenicity of the study vaccine from the two previous phases. At this point, scientists are now ready to show that those vaccinated with the study vaccine will unlikely contract the disease as compared to those who did not get the vaccine.
The duration of the study is usually six to 12 months. However, it can extend even longer depending on whether the number of subjects contracting the disease (both vaccinated and unvaccinated) has not been reached. There is always a follow-up period of six to 12 months for investigators to look and discover subjects who would contract the disease and test them sufficiently without knowing who were vaccinated and who were not. Upon termination of the study, the efficacy of the vaccine is established by a positive result that the percentage of vaccinated subjects DID NOT contract the disease compared to those who were not vaccinated.
“The phase III clinical trial is the pivotal study on which licensing is based and sufficient data have to be obtained to demonstrate that a new product is safe and effective for the purpose intended.” (7)
During the early part of Phase 3 (termed as Phase 3-A) human clinical trials or about six to 12 months after commencement and with a noteworthy efficacy, the vaccine may be applied for licensure. This is regardless of the fact that Phase 3 continues for another two or more years toward the follow-up period (Phase 3-B).
A company may choose to apply for licensure based on the safety and efficacy data presented and the study report will be presented to regulatory bodies such as the Food and Drug Administration. (FDA). The FDA has the power to issue the Certificate of Registration (CFR) after thoroughly evaluating and approving the results of the clinical trials.
With a CFR from FDA, a vaccine can then be made available, sold and disseminated for public use. Dengvaxia, the dengue vaccine followed this path and was approved for use by the FDA in December 2015. After four months it was administered among grade 4 students nine years and older to control and prevent dengue.

A Phase 4 called the Post- Marketing Surveillance follows with the vaccine’s public use. This is intended to further prove the efficacy of the FDA approved vaccine and monitor significant incidents arising from its public use. Strictly speaking this is not necessarily part of the trial. However, this is a requirement for new vaccines to insure that expanded use can still be studied further and to see if there are any rare or very rare adverse events that may occur when a vaccine is used in hundreds of thousands or millions of doses.
MOST SOUGHT VACCINE WORLDWIDE
At the forefront of the search for the COVID-19 vaccine is the WHO. It is now leading countries to form a coalition to fast-track the development of a COVID-19 vaccine that will be safe and efficacious for distribution in all countries, with consideration of equity and appropriate distribution and delivery mechanisms to prioritize those most in dire need. Because some countries have higher incidence than others and the state of their economies are far and wide, access to an effective COVID vaccine may not be readily available to those who need it most.
Funds to get the vaccines when they become available may be good for first world countries while much inadequate for other impoverished states. Moreover, the huge possibility of the so-called vaccine nationalism will put the limited available vaccines only for their own use defeating the objective of global distribution. There will be different scenarios in the future, but WHO maintains that when a safe and effective vaccine is found, COVAX, a coalition led by WHO, the Global Alliance for Vaccine Initiatives (GAVI) and the Coalition for Epidemic Preparedness Innovations (CEPI) will facilitate the equitable access and distribution of these vaccines to protect people in all countries. As far as the world health body is concerned. It is committed to provide the vaccine to people most at risk first.
Currently, governments and independent private companies are developing the COVID-19 vaccine. Most advanced and in Phase 3 is the United States with Pfizer and Moderna announcing they have completed recruitment of 30,000 subjects. Johnsons and Johnsons with one dose has also entered into phase 3, resuming again after a temporary stop when a subject became ill. AstraZeneca in the UK also had a temporary disruption but is now continuing and is also in an advanced state of phase 3A.

Russia and China, which licensed their vaccine after Phase 2 only, are using the vaccine publicly on their citizens. Sinovac in China has been giving their 2-dose vaccine for about US$60 since mid-October 2020, according to a CNN report. It is unsure when a vaccine from other countries like China which has not yet been completely tested will be made available to the Philippines. Philippine FDA will be responsible for making sure that the vaccines to be used in the country have undergone standard scientific procedures to ensure safety and efficacy.
The Philippines has joined the more than 90 countries now engaged in WHO solidarity trials both for therapeutic drugs and vaccines for COVID-19. The Department of Science and Technology (DOST) has announced that they have started to look into the first two vaccine trials for Phase 3 that may be started as soon as the requirements have been submitted by the sponsoring companies. The FDA announced that they will try to facilitate the procedures so as to start these trials before the end of the year or early next year. The approval for each step of the process may take several weeks and will not be possible until after another month or two.
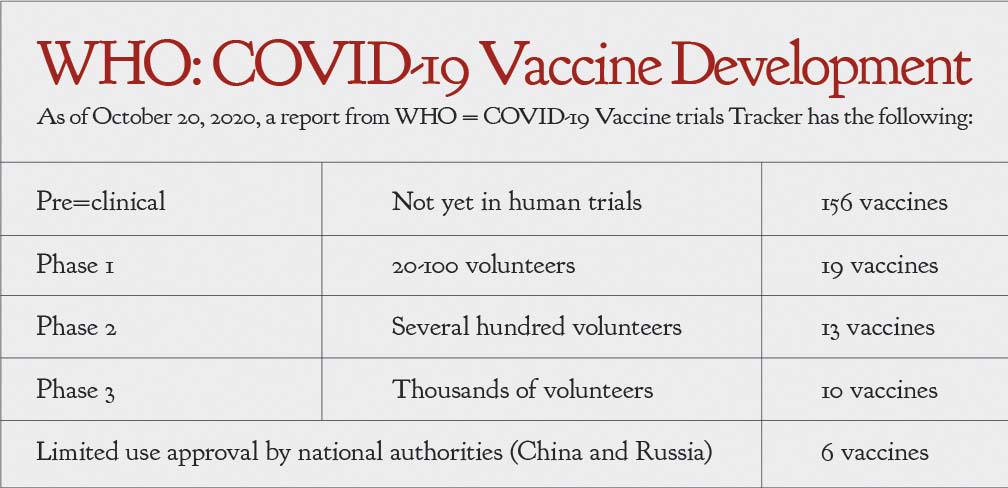
As this unfolds, if Phase 3 will be undertaken, we can expect a vaccine to be available for licensure if successful only after 6-12 months. However, no vaccine trial in the Philippines has started and no definite date has yet been set. With so many steps toward development discussed earlier, a Philippine COVID-19 vaccine for Filipinos is still farfetched. There are just too many processes still needing to be done with ethical reviews and budget considerations presenting the biggest stumbling blocks.
IMPEDIMENTS TO VACCINE UTILIZATION
Despite their doubtless benefits to mankind, vaccines and vaccination often encounter fierce public resistance.
The presence of anti-vaccination groups and vaccine resistance movements in various parts of the globe have always been part of vaccine development history.

Numerous and increasing numbers of legal cases and corresponding litigation have affected vaccine development, use and manufacturers’ profitability through the years.
Political flavor and marked promotion of vested interests by groups and countries are stark realities of today’s digital communication age.
Thus, it is now a fact that companies involved in vaccine manufacturing have decreased through the years specially in the last quarter of the 20th century.
Concurrently however, the passage and implementation of the National Vaccine Injury Compensation Programme in the US in 1986 also served to renew interests in vaccine development and led to application of molecular genetics and heightened researches on immunology and genomics. This has significantly increased in the last three decades.

PHILIPPINE IMMUNIZATION PROGRAM
With every human on earth today pinning hopes for an effective and safe COVID-19 vaccine, realists in the medical field know and state that COVID-19, being novel as designated, will eventually be made but not without obstacles mentioned previously as well as new and yet undiscovered scenarios.
Adequate preparation is the all important key. The dengue controversy: Philippine Experience is a case in point.
What started as a questioned specific single health program of the past administration turned into a highly politicized controversy promoted by certain individuals for their perceived ends.
It gave rise to grave misinformation and unfounded holistic fear of vaccine use. This greatly impeded the national immunization program of the Philippines and drove it back by decades.
But the dengue scare that ensued must never be triggered again. Still a lingering nightmare at present, it has become the finest example of how a specific successful health system is unreasonably driven astray.
Hysteria arising from an unfortunate mix of events, personalities and agendas, beclouded by political colors, tainted by arrogance, ambition and unfounded claims and hearsay altogether crushed Filipinos’ public confidence and trust in all vaccines and government-led health programs.
Of course, the greatest casualties are the Filipino children, left vulnerable to otherwise controlled and controllable diseases such as measles and polio, because the false hype created by the controversy scared the inadequately-informed parents of the value of vaccines altogether.
Alongside the Filipino scientists, are the countless men and women of the health care delivery system who were just performing their avowed jobs but were also severely affected by the misinformation that media proliferated.
The controversy involving but a single specific vaccine produced a tragic domino effect, driving down total immunization coverage rates to unimaginable depths.
In a study done by WHO in selected areas in Metro Manila in 2018, the measles outbreak was attributed to vaccine hesitancy when parents stopped and shunned scheduled immunizations of their children for fear that vaccines are unsafe due to the Dengvaxia fiasco. (8)
Clearly, there is a desperate need to address targeted solutions to factors that drive this resultant vaccine hesitancy.
The Philippine health care system now must adopt and propagate the so-called 3C’s model. Prioritizing the restoration of Confidence defined as trust in the effectiveness and safety of vaccines, in the system that delivers them, and the motivations of policy-makers (9) tackling, Complacency brought about by low perceived risks to a vaccine preventable disease with more of life’s responsibilities taking precedence and Convenience which includes practical issues as affordability, logistics and geographical reach among others.
THE WAY FORWARD: VACCINE RESILIENCY

From the Philippine experience, it becomes clear that any ideal immunization program aimed at eradicating preventable disease must have proper safeguards built in. Such safeguards ensure the programs’ continuity and persistence in the face of unexpected situations.
Thus the concept of Vaccine Resiliency becomes all the more important for consideration. It may be defined as the ability of a vaccination program to withstand major shocks and disruptions by enabling the program to immediately address unforeseen circumstances; and resolve them outright.
Proper measures are timely adopted by the program to shield itself from actual and potential problems that weaken its acceptance. And even though unusual events derail or dampen implementation, popular acceptance and patronage will not be seriously affected.
We have seen this concept of Vaccine Resiliency in the successful immunization programs of countries like Singapore, Thailand, Malaysia, Korea and Japan who were all able to bounce back from the debilitating effects of COVID-19.
Had the Philippine program been as vaccine resilient as the aforementioned Asian countries, many more lives would have been saved.
The personal and global commitment to save children’s lives by immunization is the goal but the dedicated and sustained commitment is still highly inadequate to date.
In 2011, the decade of the Vaccine was announced by the WHO designating 2011-2020 as the Decade of the Global Vaccine Action Plan (GVAP). It essentially aimed to increase immunization services and accessibility, and introduce new vaccines to save countless young individuals.
The GVAP is coupled with continuing programs of vaccine research and development. But as it draws to a close this year, the ten-year Plan has not adequately delivered as envisioned. The need therefore, of a more thorough and comprehensive successor Plan aiming for advocates and partners become an imperative of our time.
It is for the sake of the Filipinos, young and old alike to work together to restore and sustain vaccine confidence and make the country vaccine resilient. Failure to do so brings the country back to where it was 25 years ago.
References:
1. https://www.cdc.gov/smallpox/history//html
2. https://www.who.int/about/bugs_drugs_smoke_chapter_1_smallpox
3. Red Book 2018 -2021, Third Edition. Report of the Committee on Infectious Diseases. American Academy of Pediatrics.
4. https://www.cdc.gov/smallpox/clinicians/clinical-disease.html
5. Boylston, Arthur. The origins of inoculation .J R Soc Med 2012: 105(7 ): 309–313.
6. Behbehani, Abbas. The Smallpox Story: Life and Death of an Old Disease. Microbiological Reviews. Dec 1983. 455-509
7. https://www.who.int/biologicals/publications/clinical_guidelines_ecbs_2001.
8. https//www.doh.gov.phnode
9. MacDonald, N.E .Vaccine Hesitancy: Definition,scope and determinants. Vaccine 33 (2015) 4161–4164.